Artemis Data Book
2.9 Fusion Power from the Moon
Lunar Helium-3 as an Energy Source, in a nutshell
The Setting
For the purposes of this discussion, let’s assume that the helium-3 fusion plants have been proved out, and folks are frantically building them, just waiting for us to show up with with tanks full of He³.
The Ingredients of Nuclear Fusion
The names of the ingredients for nuclear fusion reactions — deuterium, tritium, isotopes of helium, sound complicated, but really these are only variations on substances found in everyday life. We’ll assume you understand that atoms are made up of protons, neutrons, and electrons.Electons是非常轻量级的,带负电荷的位that buzz around the edges of an atom. Regular chemical reactions work by trading and sharing electons among atoms. For instance, when you burn a piece of paper in air, the chemical reaction involves some carbon atoms in the paper sharing electrons with some oxygen atoms from the air. The reaction forms carbon dioxide gas while the electons give off energy, as heat and light, when they change their orbits.
Protonsandneutronsmake up an atom’s nucleus. In this discussion, we’re concerned with rearranging the nucleus of an atom; hence the term “nuclear reaction.” Generally, the neutrally charged neutrons keep the positively charged protons from fighting each other. An atom’s nucleus is very tightly bound together, so when we start moving these things around, we’re moving energy around in a big way.
Hydrogenis the familiar stuff used to make up water by combining it with oxygen. It’s the most abundant element in the universe. Normal hydrogen has 1 proton and no neutrons.氘is an isotope of hydrogen that has a neutron next to its lonely proton.
You’re familiar withheliumgas as the stuff we use to blow up blimps and balloons. Normal helium has 2 protons and 2 neutrons in its nucleus, giving it an atomic weight of 4.
Now, if you kick out one of neutrons, you get helium-3. This happens once in a while in very energetic nuclear reactors, especially the sun. The sun produces helium by fusing hydrogen atoms together, but about one in every ten thousand helium atoms comes out missing a neutron.
Helium-3 casts lustful eyes upon that neutron in the deuterium, and will grab it if it gets a chance. We give it a chance by introducing the helium-3 to the deuterium at a high temperature.
The Mixture
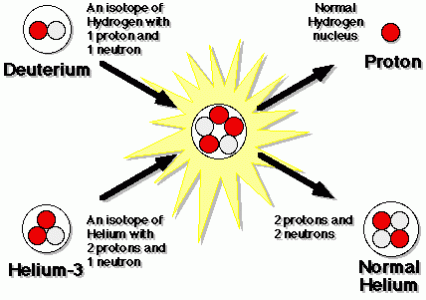
Helium-3 is used in a reaction with deuterium to produce energy:
这是一个核聚变反应。氘的nd helium-3 atoms come together to give off a proton and helium-4. The products weigh less than the initial components; the missing mass is converted to energy. 1 kg of helium-3 burned with 0.67 kg of deuterium gives us about 19 megawatt-years of energy output.
The fusion reaction time for the D«»He³ reaction becomes significant at a temperature of about 10 KeV, and peaks about about 200 KeV. A 100 KeV, or so, reactor looks about optimum.
A reactor built to use the D«»He³ reaction would be inherently safe. The worst case failure scenario would not result in any civilian fatalities or significant exposures to radiation.
Note:MeVandKeVare measures of energy, standing for mega-electron volts and kilo-electron volts, respectively. In nuclear physics, these terms are used to refer to the amount of energy in a nuclear reactor. One electron volt is the energy acquired by one electron falling through a potential of one volt, equal to approximately 1.609 E-19 joule.
The supply
Some helium-3 is available on Earth. It is a by-product of the maintenance of nuclear weapons, which would supply us with about 300 kg of helium-3 and could continue to produce about 15 kg per year. The total supply in the U.S. strategic reserves of helium is abou 29 kg, and another 187 kg is mixed up with the natural gas we have stored; these sources are not renewable at any significant rate.
In their 1988 paper, Kulcinski, et al. (see ref note below), estimate a total of 1,100,000 metric tonnes of helium-3 have been deposited by the solar wind in the lunar regolith. Since the regolith has been stirred up by collisions with meteorites, we’ll probably find helium-3 down to depths of several meters.
The highest concentrations are in the lunar maria; about half the helium-3 is deposited in the 20% of the lunar surface covered by the maria.
To extract helium-3 from the lunar soil, we heat the dust to about 600 degrees C.
We get most of the other volatiles out at the same time, so we’ll be heating up the rocks anyway. To get the oxgyen out, we’ll turn up the furnace to about 900 deg C and do some other nasty stuff; but that’s a different story.
The Energy
That 1 million metric tonnes of helium-3, reacted with deuterium, would generate about 20,000 terrawatt-years of thermal energy. The units alone are awesome: a terrawatt-year is one trillion,
10 to 12th power watt-years. To put this into perspective, one 100-watt light bulb will use 100 watt-years of energy in one year.
That’s about 10 times the energy we could get from mining all the fossil fuels on Earth, without the smog and acid rain. If we torched all our uranium in liquid metal fast breeder reactors, we could generate about half this much energy, and have some interesting times storing the waste.
The Value
About 25 tonnes of helium-3 would power the United States for 1 year at our current rate of energy consumption. To put it in perspective: that’s about the weight of a fully loaded railroad box car, or a maximum Space Shuttle payload.
To assign an economic value, suppose we assume helium-3 would replace the fuels the United States currently buys to generate electricity. We still have all those power generating plants and distribution network, so we can’t use how much we pay for electricity. As a replacement for that fuel, that 25-tonne load of helium-3 would worth on the order of $75 billion today, or $3 billion per tonne.
回报
A guess is the best we can do. Let’s suppose that by the time we’re slinging tanks of helium-3 off the moon, the world-wide demand is 100 tonnes of the stuff a year, and people are happy to pay $3 billion per tonne. That gives us gross revenues of $300 billion a year.
To put that number in perspective: Ignoring the cost of money and taxes and whatnot, that rate of income would launch a moon shot like our reference mission every day for the next 10,000 years. At that point, we will have used up all the helium-3 on the moon and had better start thinking about something else.
Reference: Kulcinksi, Cameron, Santarius, Sviatoslavsky, and Wittenberg, “Fusion Energy from the Moon for the 21st Century.” 1988. Fusion Technology Institute, University of Wisconsin.
For more information: Helium-3 as a Source of Energy for the 21rst Century.